Arjumand Ghazi, PhD
Principal Investigator
arjumand.ghazi@chp.edu
Read more »
Francis Amrit Gandhi, PhD
Post-doctoral Fellow
francis.gandhi@chp.edu
Hye Jin Hwang, PhD
Post-doctoral Scholar
hyejin.hwang@chp.edu
Rachana Kelkar, BS
Graduate Student
rsk43@pitt.edu
Julia A. Loose, BS
Graduate Student
jal260@pitt.edu
Nikki Naim, BS
Graduate Student
nin27@pitt.edu
Thayjas Patil
Undergraduate Researcher
tap97@pitt.edu
Contact Us
The Ghazi Laboratory is located at:
John G. Rangos Sr. Research Center
Room 7139
One Children's Hospital Drive
4401 Penn Avenue
Pittsburgh, PA 15224
To reach the lab by phone please call 412-692-9258.
For administrative matters, please contact Teresa Trimble at trimble2@chp.edu or 412-692-5412.
Alumni
- Ramya Mallela, Technician, 2015-2016, Graduate student, University of Pittsburgh
- Ramesh Ratnappan, Post-doctoral Fellow, 2012-2015, Post-doctoral fellow, George Washington University
- Scott A. Keith, Technician, 2013-2015, Graduate student, Carnegie Mellon University
- Kyle Holden, Technician, 2011-2014, Medical student, Lake Erie College of Osteopathic Medicine
Undergraduate Alumni
2018 Summer Team

Francis Gandhi, Arjumand Ghazi, Dom Piganelli, Julia Loose, Nikki Naim and Hye Jin Hwang celebrating the comprehensive exam success of Julia and Nikki.
2017 Summer Team

Francis Gandhi, Nikki Naim, Arjumand Ghazi, Carter Mason, Julia Loose, Alexandra Borelli1, Joseph Garve1, and Hye Jin Hwang
2016 Summer Team

- Mark D’Alesio1, University of Pittsburgh, Spring – Summer 2016
- Sneha Rajendran2, University of Pittsburgh, Spring – Summer 2016
- Hannah Smith1, Allegheny College, Summer 2016
- Anika Fedak1, Pittsburgh Science and Technology Academy, Summer 2016
2015 Summer Team

- Nicholas DelBuono3, 5, University of Pittsburgh, Fall 2012 – Summer 2015
- Alekya Kothamasu, University of Pittsburgh, Spring 2014 – Fall 2015
- Anika Manikurve1, University of Pittsburgh, Summer – Fall 2015
- Eva Roy1, University of Pittsburgh, Summer 2015
- Levi Kalka, University of Pittsburgh, Spring 2015
2014 Summer Team

- Lia Petrose, University of Pittsburgh, Spring 2014
- Laura Steenberge4, Illinois Wesleyan University, Summer 2014
- Denise Monti1, Washington University in St. Louis, Summer 2013, Summer 2014
- Kayla Thomason1, University of Pittsburgh, Fall 2012 – Summer 2014
- Hetal Patel, University of Pittsburgh, Fall 2013
2013 Summer Team

- Martin Echavarria, University of Pittsburgh, Fall 2012
- Hasreet Gill4, Grove City College, Summer 2011
- Sarah Winston1, University of Pittsburgh, Spring – Summer 2012
- Leisha Steenkiste4, Denison University, Summer 2012
- Sarah Bass1, Pennsylvania State University, Summer 2011
Support:
1 Children’s Hospital of Pittsburgh of UPMC, Summer Fellowship
2 University of Pittsburgh, Honors College, Health Sciences Summer Fellowship
3 University of Pittsburgh, Brackenridge Fellowship
4 University of Pittsburgh, Summer Undergraduate Research Program Fellowship
5 Neuroscience honors research thesis undertaken while in the lab.










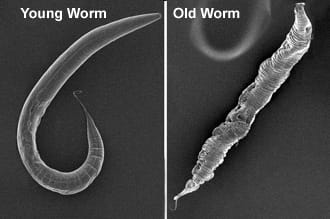 We study longevity genes in Caenorhabditis elegans, a model organism with a short lifespan of about three weeks. C. elegans displays many anatomical and functional changes that accompany human aging, as seen here in the scanning electron microscopy images of young and old C. elegans from our lab. Worm genes show strong homology with their human counterparts. Indeed, the most well known longevity pathway, the insulin/IGF1 signaling (IIS) cascade, was first identified for its role in aging in worms. Since then, many fundamental discoveries in the biology of aging have been made in C. elegans, and it continues to be a remarkably valuable system for aging research.
We study longevity genes in Caenorhabditis elegans, a model organism with a short lifespan of about three weeks. C. elegans displays many anatomical and functional changes that accompany human aging, as seen here in the scanning electron microscopy images of young and old C. elegans from our lab. Worm genes show strong homology with their human counterparts. Indeed, the most well known longevity pathway, the insulin/IGF1 signaling (IIS) cascade, was first identified for its role in aging in worms. Since then, many fundamental discoveries in the biology of aging have been made in C. elegans, and it continues to be a remarkably valuable system for aging research.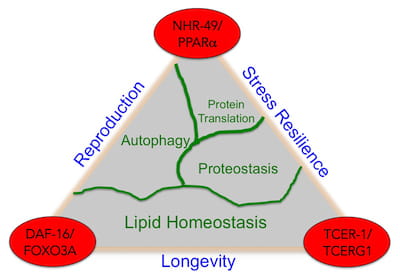 While it is well recognized that increasing age impairs reproductive fitness, how the germline of an animal influences aging of its somatic cells is not known. Recent discoveries in worms, flies and mice have shown that signals from reproductive tissues indeed influence the length and quality of life of the whole organism.
While it is well recognized that increasing age impairs reproductive fitness, how the germline of an animal influences aging of its somatic cells is not known. Recent discoveries in worms, flies and mice have shown that signals from reproductive tissues indeed influence the length and quality of life of the whole organism.
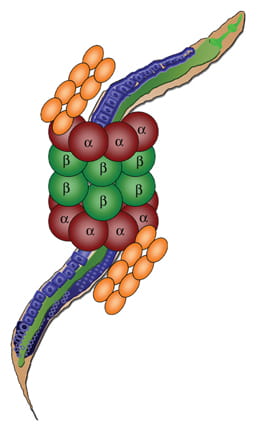 Proteasomal Regulation of Aging
Proteasomal Regulation of Aging






